Information on submitting a notification or an application for authorisation of an activity involving the contained use of genetically modified organisms, alien organisms subject to compulsory containment or pathogenic organisms.
Do you handle genetically modified organisms (GMOs), alien organisms subject to compulsory containment or pathogenic organisms (pathogenic to humans, animals or plants) in a laboratory, greenhouse, animal unit or other contained facility? Then you will have to determine whether your activity requires notification or authorisation under the Ordinance on the Contained Use of Organisms (Containment Ordinance, ContainO; SR 814.912).
The details below should help you with this. The brochure on biological experiments provides an overview of the legal requirements and their implementation (in German, translation in progress). Further information can also be found in the commentary on the Containment Ordinance.
Biologische Experimente - Schweizer Regeln, Tipps und Kontakte (PDF, 279 kB, 08.12.2020)Im Auftrag des BAFU
Guide to the notification and authorisation procedures
Alien organisms subject to compulsory containment
Small alien invertebrates, invasive alien organisms according to appendix 2 of the release ordinance and organisms considered to be especially dangerous according to the appendices 1, 2, and 6 of the ordinance on plant protection are collectively termed "alien organisms subject to compulsory containment".
There is no list of alien organisms subject to compulsory containment according to their risk classification. The procedure for the classification of those alien organisms and of activities using them is described in more detail in the commentary on the containment ordinance (German/French, p. 22, pp. 41).
What is an activity?
An activity as defined by the ContainO expressly covers only the intentional (deliberate) handling of genetically modified organisms, alien organisms subject to compulsory containment or pathogenic organisms in contained systems, and excludes mere exposure within the scope of any other work. Activities covered by the ContainO include those in which genetically modified organisms, alien organisms subject to compulsory containment or pathogenic organisms are, for instance, cultured, processed, multiplied, modified, detected, transported, stored or disposed of.
According to the ContainO, the entirety of all manipulations that form a logical unit according to type, scope and purpose (e.g. a research project for the Swiss National Science Foundation, carrying out various analyses in a diagnostics laboratory) are treated as a single activity. One activity may therefore encompass several different manipulations in which GMOs or pathogenic organisms are deliberately used. Work within different areas such as research, diagnostics and production, on the other hand, are considered separate activities.
Risk assessment
Before commencing an activity as defined by the ContainO, a risk assessment must be carried out (Art. 8 CO).
Based on the organisms to be used and taking into account the type and extent of the planned work, the person responsible for the activity must evaluate the conceivable possibilities and the consequences for humans and the environment if the organisms were to escape from the contained system. The risk assessment should also consider the efficacy of protective measures to prevent possible damage.
The classification of the organisms used according to the risk they present (see Lists of organisms) into one of four risk groups (Art. 6 ContainO) is an important aid to risk assessment.
We refer to the cell line list of the ZKBS:
The risk assessment assigns the planned activity to one of the four activity classes in accordance with Article 7 ContainO.
The risk must be reassessed if the activity is significantly revised or if significant new findings become available. A significant revision of an activity includes:
- the use of new organisms in class 3 and 4 activities;
- the use of new organisms with significantly different properties in class 1 and 2 activities (e.g. human- v. animal- or plant-pathogenic organisms; aerogenically v. non-aerogenically transmissible organisms);
- starting part of an activity in a different type of installation (see Appendix 4, Number 2 Para. 1, Letters a-d ContainO).
Significant new findings include:
- new epidemiological data on an organism;
- new data concerning the safety of a technical measure.
Recording and storage of documents
The duty to record and store documentation applies to all activities.
All documents concerning the safety of an activity must be stored and presented to the authorities for inspection at their request. These documents always include the risk assessment, and any documents submitted to the authorities in the course of notification.
Notification and authorisation of activities
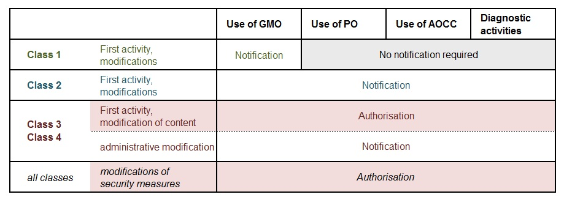
GMO: genetically modified organisms
PO: pathogenic organisms
AOCC: alien organisms subject to compulsory containment
Deadlines
Class 3 and 4 activities and the alteration, substitution or omission of particular safety measures are subject to authorisation, and may be carried out only after the competent authority (Federal Office for the Environment FOEN or Federal Office of Public Health FOPH) has granted authorisation.
The Federal Coordination Centre for Biotechnology requires around up to 20 days to check that the documentation submitted is complete and request any missing information, then around 90 days to gather opinions from the various institutions, and to evaluate the application for authorisation by the competent authority. If the examination of the submitted documents shows that they are incomplete, the procedure can take correspondingly longer. The responsible agencies do however try to keep this period as short as possible and therefore give priority to applications for authorisation.
Authorisation of a class 3 or 4 activity is valid for a maximum of five years. It can be renewed if an extension application is submitted to the Federal Coordination Centre for Biotechnology before the valid authorisation expires. If such an application is submitted in good time, the activity can be continued without interruption until the authorities' decision. The renewed authorisation is then also valid for a maximum of five years.
If a renewal application has not been submitted before the authorisation expires, the activity must cease. In order to restart the activity, a new authorisation application must be submitted.
All other notified activities that are not subject to authorisation may be commenced immediately.
Modifying submitted notifications and authorisations
Modifications with reassessment of risk (Modification of the activity)
This group includes modifications that significantly alter the activity, such as:
- the use of new organisms in class 3 and 4 activities;
- the use of new organisms with substantially different properties (e.g. human- v. animal- or plant-pathogenic organisms; aerogenically v. non-aerogenically transmissible organisms). If the omission of a safety measure has been authorised, and the safety measure is to be omitted for newly used organisms as well, then the authorisation must be extended to cover these organisms;
- starting part of an activity in a different type of installation (e.g. additional handling of animals at the same or another location).
The altered activity may commence:
- when the alteration is submitted for class 1 and 2 activities
- after receipt of authorisation for notified modifications (please note that in this case the procedure may take up to 110 days).
Modifications without reassessment of risk
This group includes administrative changes such as
- a change of Project Leader or Biosafety Officer
- a change of address for the installation, or a move to a new location
- stopping or extending the activity
- the use of additional or other non-notified premises.
The activities may be continued without interruption.
Confidential information
The public is informed about notifications and authorisation applications on the Internet and through publication in the Federal Gazette. They have the right to consult these documents at the Federal Coordination Centre for Biotechnology.
If particular information is to be treated as confidential, it may be submitted in one of the following ways:
- Two forms are submitted, the first of which contains all the information and the second only the non-confidential information.
- Only the form with the non-confidential information is submitted; the confidential information is given in a document that is submitted separately.
All documents that contain confidential information must be marked accordingly.
Fees
Federal agency services must be invoiced in accordance with the ContainO. A set fee is usually required. If extraordinary work is needed for a notification or application, the fee will be calculated according to the work involved (ContainO).
Last modification 01.06.2021





